Research Experience for Undergraduates
By: Vicki Hixon
Published Date: Jan 18
Congratulations to Drs. Pozzo-Miller and King who have received a Research Experience for Undergraduates award from the National Science Foundation. This award will allow the Department of Neurobiology to host 10 students from outside of UAB for 10 weeks of summer research training and professional development. Students who are interested in pursuing PhD training after their BS/BA should apply for the March 1, 2017 deadline. The application is online now at: http://www.uab.edu/medicine/neurobiology/education/undergraduate-summer-research
CIRC Travel Awards 2017
By: Vicki Hixon
Published Date: Jan 10
The Civitan International Research Center is announcing the availability of trainee travel awards to facilitate presentations at meetings, conferences or symposia related to research and/or clinical activities addressing neurodevelopmental disabilities. Individual awards of up to $1000 will apply toward conference registration fees, airfare, hotel and meals according to UAB travel policy.
Application deadlines will be April 1 and September 1, 2017.
Applications should include the following in a single pdf file:
· a description of the travel opportunity and relationship to the trainee program of study
· a one-page CV from the trainee
· a letter of endorsement from the mentor clearly stating how the student will benefit from participation and also that no other support is available for this travel
· travel budget details
· copy of abstract – include letter of acceptance
The mission of the CIRC is to improve the well-being and the quality of life of individuals and families affected by neurodevelopmental disabilities; to provide interdisciplinary clinical and research training in neurodevelopmental disabilities; to utilize this knowledge to develop and provide high quality exemplary services and programs; and to exchange information in a timely way with consumers, practitioners, scientists, and society.
Submission guidelines:
Title the subject matter: Travel Application "Last name"
Submit to:
Vicki Hixon vhixon@uab.edu
The Civitan International Research Center is announcing the availability of trainee travel awards to facilitate presentations at meetings, conferences or symposia related to research and/or clinical activities addressing neurodevelopmental disabilities. Individual awards of up to $1000 will apply toward conference registration fees, airfare, hotel and meals according to UAB travel policy.
Application deadlines will be April 1 and September 1, 2017.
Applications should include the following in a single pdf file:
· a description of the travel opportunity and relationship to the trainee program of study
· a one-page CV from the trainee
· a letter of endorsement from the mentor clearly stating how the student will benefit from participation and also that no other support is available for this travel
· travel budget details
· copy of abstract – include letter of acceptance
The mission of the CIRC is to improve the well-being and the quality of life of individuals and families affected by neurodevelopmental disabilities; to provide interdisciplinary clinical and research training in neurodevelopmental disabilities; to utilize this knowledge to develop and provide high quality exemplary services and programs; and to exchange information in a timely way with consumers, practitioners, scientists, and society.
Submission guidelines:
Title the subject matter: Travel Application "Last name"
Submit to:
Vicki Hixon vhixon@uab.edu
Jeremy Day Probes Reward Signaling in the Brain
By: Vicki Hixon
Published Date: Jan 03
The Scientist - January 2017 Edition - Exploring Life, Inspiring Innovation
www.The-scientist.com
SCIENTIST TO WATCH Assistant Professor, University of Alabama at Birmingham. Age: 35
BY CATHERINE OFFORD

Jeremy Day: Reward Researcher
As an undergraduate at Auburn University in the early 2000s, Jeremy Day was thinking of becoming an architect. But an opportunity to work on a research project investigating reward learning in rodents changed the course of his career. “It really hooked me,” he says. “It made me immediately wonder what mecha- nisms were underlying that behavior in the animal’s brain.”
It’s a question Day has pursued ever since. In 2004, he enrolled in a PhD program at the University of North Carolina at Chapel Hill and began studying neural reward signaling under the mentorship of neu- roscientist Regina Carelli. “He was a stellar student by all accounts,” Carelli recalls. “He was very clear on the type of work he wanted to do, even that early on in his career.” Focusing on the nucleus accum- bens, a brain region involved in associative learning, Day measured dopamine levels in rats undergoing stimulus-reward experiments. Although a rat’s brain released dopamine on receipt of a reward early in training, Day found that, as the rodent became accustomed to spe- cific cues predicting those rewards, this dopamine spike shifted to accompany the cues instead, indicating a changing role for the chemical during learning.1
Day completed his PhD in 2009, but realized that to better understand dopamine signaling and errors in the brain’s reward system that lead to addiction, he would need a broader skill set. “I had a strong background in systems neuroscience, but my training in molecular neuroscience was not as strong,” he explains. So he settled on “a field that I knew almost nothing about”—epigenetics—and joined David Sweatt’s lab at the University of Alabama at Birmingham (UAB) as a postdoc. For someone used to a field where “data come in as it’s happening,” Day says, “transitioning to a molecular lab where you might do an assay and you don’t get an answer for a week or two was a culture shock.”
Initially, Day investigated epigenetic modification in the nucleus accumbens. “The idea was that we’d block DNA methylation and see if we could also block learning,” he explains. But things didn’t go according to plan. “We worked on that for a couple of years and, basically, all the results from that experiment were negative.”
Instead of giving up, Day refocused. “He demonstrated a lot of perseverance,” recalls Sweatt. “It really took commitment and determination to stick with the project.” Leaving the nucleus accumbens, Day tried similar experiments in another site involved in dopaminergic pathways. “We found that if we blocked DNA methylation in that region, we could completely block an animal’s ability to learn about rewards,” Day says.2
In 2013, Day received a grant from the National Institute on Drug Abuse (NIDA) that helped him set up as an assistant professor at UAB the following year. He has continued collaborating with Sweatt—now at Vanderbilt University School of Medicine—who calls his former postdoc “a rising star in the discipline.” In 2016, they published evidence that extra-coding RNAs—noncoding RNAs whose sequences overlap with protein- coding regions—help regulate neuronal DNA methylation in an activity-dependent manner.3
Now, Day is most excited about CRISPR-Cas9’s potential to explore epigenetics in the brain. “For the first time, we have the ability to look at the causal role for these modifications in gene regulation, neural function, and behavior,” he says. “It’s a really fun time to be in the field.” J
REFERENCES 1. J.J. Day et al., “Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens,” Nat Neurosci, 10:1020-28, 2007. (Cited 359 times) 2. J.J. Day et al., “DNA methylation regulates associative reward learning,” Nat Neurosci, 16:1445-52, 2013. (Cited 82 times) 3. K.E. Savell et al., “Extra-coding RNAs regulate neuronal DNA methylation dynamics,” Nat Commun, 7:12091, 2016. (Cited 1 time)
www.The-scientist.com
SCIENTIST TO WATCH Assistant Professor, University of Alabama at Birmingham. Age: 35
BY CATHERINE OFFORD

Jeremy Day: Reward Researcher
As an undergraduate at Auburn University in the early 2000s, Jeremy Day was thinking of becoming an architect. But an opportunity to work on a research project investigating reward learning in rodents changed the course of his career. “It really hooked me,” he says. “It made me immediately wonder what mecha- nisms were underlying that behavior in the animal’s brain.”
It’s a question Day has pursued ever since. In 2004, he enrolled in a PhD program at the University of North Carolina at Chapel Hill and began studying neural reward signaling under the mentorship of neu- roscientist Regina Carelli. “He was a stellar student by all accounts,” Carelli recalls. “He was very clear on the type of work he wanted to do, even that early on in his career.” Focusing on the nucleus accum- bens, a brain region involved in associative learning, Day measured dopamine levels in rats undergoing stimulus-reward experiments. Although a rat’s brain released dopamine on receipt of a reward early in training, Day found that, as the rodent became accustomed to spe- cific cues predicting those rewards, this dopamine spike shifted to accompany the cues instead, indicating a changing role for the chemical during learning.1
Day completed his PhD in 2009, but realized that to better understand dopamine signaling and errors in the brain’s reward system that lead to addiction, he would need a broader skill set. “I had a strong background in systems neuroscience, but my training in molecular neuroscience was not as strong,” he explains. So he settled on “a field that I knew almost nothing about”—epigenetics—and joined David Sweatt’s lab at the University of Alabama at Birmingham (UAB) as a postdoc. For someone used to a field where “data come in as it’s happening,” Day says, “transitioning to a molecular lab where you might do an assay and you don’t get an answer for a week or two was a culture shock.”
Initially, Day investigated epigenetic modification in the nucleus accumbens. “The idea was that we’d block DNA methylation and see if we could also block learning,” he explains. But things didn’t go according to plan. “We worked on that for a couple of years and, basically, all the results from that experiment were negative.”
Instead of giving up, Day refocused. “He demonstrated a lot of perseverance,” recalls Sweatt. “It really took commitment and determination to stick with the project.” Leaving the nucleus accumbens, Day tried similar experiments in another site involved in dopaminergic pathways. “We found that if we blocked DNA methylation in that region, we could completely block an animal’s ability to learn about rewards,” Day says.2
In 2013, Day received a grant from the National Institute on Drug Abuse (NIDA) that helped him set up as an assistant professor at UAB the following year. He has continued collaborating with Sweatt—now at Vanderbilt University School of Medicine—who calls his former postdoc “a rising star in the discipline.” In 2016, they published evidence that extra-coding RNAs—noncoding RNAs whose sequences overlap with protein- coding regions—help regulate neuronal DNA methylation in an activity-dependent manner.3
Now, Day is most excited about CRISPR-Cas9’s potential to explore epigenetics in the brain. “For the first time, we have the ability to look at the causal role for these modifications in gene regulation, neural function, and behavior,” he says. “It’s a really fun time to be in the field.” J
REFERENCES 1. J.J. Day et al., “Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens,” Nat Neurosci, 10:1020-28, 2007. (Cited 359 times) 2. J.J. Day et al., “DNA methylation regulates associative reward learning,” Nat Neurosci, 16:1445-52, 2013. (Cited 82 times) 3. K.E. Savell et al., “Extra-coding RNAs regulate neuronal DNA methylation dynamics,” Nat Commun, 7:12091, 2016. (Cited 1 time)
Global Study on Rett Syndrome
By: Vicki Hixon
Published Date: Dec 19
UAB part of global study of breathing issues in Rett syndrome
by Bob Shepard December 16, 2016
December 16, 2016 Print Email
December 16, 2016
UAB will study a drug originally developed for Parkinson’s disease that may help reduce breath holding in patients with Rett syndrome.
 Pediatric neurologist Alan Percy, M.D., is a leading clinician and researcher into Rett Syndrome Researchers at the University of Alabama at Birmingham are part of the international STARS study to see if a drug originally developed for Parkinson’s disease might help reduce breathing issues common in patients with Rett syndrome. The drug, sarizotan, may help reduce the frequency of breath holding, a potentially significant effect of Rett syndrome.
Pediatric neurologist Alan Percy, M.D., is a leading clinician and researcher into Rett Syndrome Researchers at the University of Alabama at Birmingham are part of the international STARS study to see if a drug originally developed for Parkinson’s disease might help reduce breathing issues common in patients with Rett syndrome. The drug, sarizotan, may help reduce the frequency of breath holding, a potentially significant effect of Rett syndrome.
“We often see Rett patients holding their breath for long periods of time, up to 30 seconds at a time, behavior that can go on for hours,” said Alan Percy, M.D., professor of neurology in the Department of Pediatrics, medical director of the UAB Civitan International Research Center and a leading Rett syndrome expert. “Patients often end up swallowing large amounts of air, which can have a very detrimental effect on nutrition, a major issue for Rett patients.”
The study, to be held in four sites in the United States and several international locations, is looking to enroll patients with Rett syndrome who are 13 years and older, have a body weight of at least 55 pounds and experience multiple episodes of breath holding while awake during the day.
Percy says UAB is looking to enroll 10-15 patients locally. Participants will be followed for one year.
“Breath holding can be quite disruptive in younger Rett patients, although it usually subsides in early adulthood,” Percy said. “We currently do not have an effective medication that addresses breath holding. This is the first multisite trial of a potential therapy.”
While its mechanism of action remains unclear, Percy says sarizotan may help prevent breath holding by activating serotonin 1a receptors in the brain stem.
“While this is certainly not curative for Rett syndrome, it could be disease modifying,” Percy said. “This has the potential to be an important drug, as breath holding can be very disruptive and distressing to the family.”
The National Institutes of Health defines Rett syndrome as a neurodevelopmental disorder that affects girls almost exclusively. It is characterized by normal early growth and development followed by a slowing of development, loss of purposeful use of the hands, distinctive hand movements, slowed brain and head growth, problems with walking, seizures, and intellectual disability.
The study is sponsored by Newron Pharmaceuticals U.S., Inc. Other study sites in the United States are the Altman Clinical and Translation Research Institute, University of California San Diego; Rush Medical University Center, Chicago; and Texas Children’s Hospital, Houston.
by Bob Shepard December 16, 2016
December 16, 2016 Print Email
December 16, 2016
UAB will study a drug originally developed for Parkinson’s disease that may help reduce breath holding in patients with Rett syndrome.
 Pediatric neurologist Alan Percy, M.D., is a leading clinician and researcher into Rett Syndrome Researchers at the University of Alabama at Birmingham are part of the international STARS study to see if a drug originally developed for Parkinson’s disease might help reduce breathing issues common in patients with Rett syndrome. The drug, sarizotan, may help reduce the frequency of breath holding, a potentially significant effect of Rett syndrome.
Pediatric neurologist Alan Percy, M.D., is a leading clinician and researcher into Rett Syndrome Researchers at the University of Alabama at Birmingham are part of the international STARS study to see if a drug originally developed for Parkinson’s disease might help reduce breathing issues common in patients with Rett syndrome. The drug, sarizotan, may help reduce the frequency of breath holding, a potentially significant effect of Rett syndrome.“We often see Rett patients holding their breath for long periods of time, up to 30 seconds at a time, behavior that can go on for hours,” said Alan Percy, M.D., professor of neurology in the Department of Pediatrics, medical director of the UAB Civitan International Research Center and a leading Rett syndrome expert. “Patients often end up swallowing large amounts of air, which can have a very detrimental effect on nutrition, a major issue for Rett patients.”
The study, to be held in four sites in the United States and several international locations, is looking to enroll patients with Rett syndrome who are 13 years and older, have a body weight of at least 55 pounds and experience multiple episodes of breath holding while awake during the day.
Percy says UAB is looking to enroll 10-15 patients locally. Participants will be followed for one year.
“Breath holding can be quite disruptive in younger Rett patients, although it usually subsides in early adulthood,” Percy said. “We currently do not have an effective medication that addresses breath holding. This is the first multisite trial of a potential therapy.”
While its mechanism of action remains unclear, Percy says sarizotan may help prevent breath holding by activating serotonin 1a receptors in the brain stem.
“While this is certainly not curative for Rett syndrome, it could be disease modifying,” Percy said. “This has the potential to be an important drug, as breath holding can be very disruptive and distressing to the family.”
The National Institutes of Health defines Rett syndrome as a neurodevelopmental disorder that affects girls almost exclusively. It is characterized by normal early growth and development followed by a slowing of development, loss of purposeful use of the hands, distinctive hand movements, slowed brain and head growth, problems with walking, seizures, and intellectual disability.
The study is sponsored by Newron Pharmaceuticals U.S., Inc. Other study sites in the United States are the Altman Clinical and Translation Research Institute, University of California San Diego; Rush Medical University Center, Chicago; and Texas Children’s Hospital, Houston.
Parpura invited to join Dana Alliance
By: Vicki Hixon
Published Date: Dec 13
UAB’s Parpura invited to join Dana Alliance for Brain Initiatives
by Bob Shepard - December 09, 2016

UAB neurobiology professor Vladimir Parpura becomes the second UAB faculty with membership in the Dana Alliance for Brain Initiatives.
by Bob Shepard - December 09, 2016

UAB neurobiology professor Vladimir Parpura becomes the second UAB faculty with membership in the Dana Alliance for Brain Initiatives.
Vladimir Parpura, M.D., Ph.D., a professor in the Department of Neurobiology (http://www.uab.edu/medicine/neurobiology/) in the School of Medicine(http://www.uab.edu/medicine/home/) at the University of Alabama at Birmingham (http://www.uab.edu/home/), has been invited to become a member of the Dana Alliance for Brain Initiatives (http://www.dana.org/About/DABI/). The Dana Alliance is a nonprofit organization whose mission is to advance public education about the progress and benefits of brain research and to disseminate information on the brain in an understandable and accessible fashion. Parpura will be only the second member of the UAB faculty with membership in the Dana Alliance, joining James MeadorWoodruff, M.D., chair of the Department of Psychiatry. There are more than 550 neuroscientists worldwide in the organization.
Outreach efforts of the Dana Alliance include organizing public forums such as an aging series targeted to older adults, facilitating speaking engagements, and supplementing K12 neuroscience curricula with publications and teaching materials through free, downloadable materials on the alliance website. The alliance’s flagship event is the annual Brain Awareness Week Campaign, now in its 22nd year. The upcoming campaign is March 1319, 2017.
Parpura earned his medical degree from the University of Zagreb in Croatia in 1989, and a doctorate in neuroscience and zoology from Iowa State University in 1993. He was elected as a Member of Academia Europaea in 2012, and has held faculty appointments at Iowa State University, the University of California, Riverside, and the University of Rijeka, Croatia, before joining UAB.
Outreach efforts of the Dana Alliance include organizing public forums such as an aging series targeted to older adults, facilitating speaking engagements, and supplementing K12 neuroscience curricula with publications and teaching materials through free, downloadable materials on the alliance website. The alliance’s flagship event is the annual Brain Awareness Week Campaign, now in its 22nd year. The upcoming campaign is March 1319, 2017.
Parpura earned his medical degree from the University of Zagreb in Croatia in 1989, and a doctorate in neuroscience and zoology from Iowa State University in 1993. He was elected as a Member of Academia Europaea in 2012, and has held faculty appointments at Iowa State University, the University of California, Riverside, and the University of Rijeka, Croatia, before joining UAB.
Four New NSF Grants.....
By: Vicki Hixon
Published Date: Dec 13
Four new NSF grants — three in neuroscience — deepen UAB’s research portfolio, forge collaborations by Jeff Hansen
Alabama now has more EPSCoR Track II grants than any other state following the award of basic science grants meant to stimulate competitive research in regions of the country traditionally less able to compete for such research funds.
Four teams of University of Alabama at Birmingham researchers have been awarded National Science Foundation grants totaling $5.4 million meant to stimulate competitive research in regions of the country that are less able to compete for these research funds.
One research team supported by an NSF grant is in the College of Arts and Sciences’ Department of Chemistry, led by a polymer chemist who applies nanotechnology to biological and biomedical challenges. The three other grants will support basic neuroscience studies, one of UAB’s hallmark research strengths.
Lori McMahon, Ph.D., the Jarman F. Lowder Professor of Neuroscience, dean of the UAB Graduate School and director of the UAB Comprehensive Neuroscience Center, highlighted the three neuroscience EPSCoR grants at this fall’s Comprehensive Neuroscience Center retreat, calling them prestigious and competitive.
“UAB neuroscience has never had one NSF grant, and now we have three,” she said. “With the results of these grants, we can increase our funding beyond the National Institutes of Health.”
These four UAB grants, and one additional Alabama-related EPSCoR that supports research teams at the University of Alabama, Tuscaloosa, and the University of Mississippi, set a record, says Christopher Lawson, Ph.D., executive director of the Alabama EPSCoR program and a professor in the UAB Department of Physics.
“The state of Alabama now has more of the EPSCoR Track II grants than any other state; no state has ever had five.”
Only 25 states, two territories and one commonwealth — areas that receive much less NSF funding than the major research universities in the other 25 states — qualify to compete for the Experimental Program to Stimulate Competitive Research grants, known as EPSCoR. The EPSCoR Track II grants are meant to level the playing fields among have and have-not research states, and applicants must form collaborations with researchers from other EPSCoR states. This means the UAB teams have formed synergistic partnerships and collaborations across the Birmingham campus and with scientists in other states to foster regional research strength.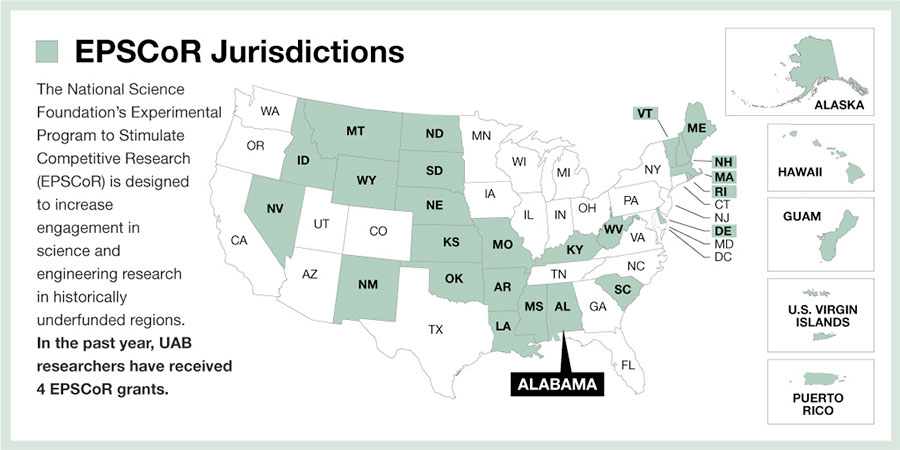
“Several institutions reached out to UAB because we are so strong in neuroscience,” McMahon said. “There is a lot of UAB synergy around the three neuroscience EPSCoRs.”
McMahon says all three UAB neuroscience teams will meet regularly to share results and ideas.
 From left: Roy Martin, Jerzy P. Szaflarski and Timothy Gawne.The current approach for epilepsy surgery at UAB involves two surgeries. The first implants electrodes into a patient’s brain for a two-week period to map the location of the seizure onset zone in the brain. The second operation cuts out the onset zone.
From left: Roy Martin, Jerzy P. Szaflarski and Timothy Gawne.The current approach for epilepsy surgery at UAB involves two surgeries. The first implants electrodes into a patient’s brain for a two-week period to map the location of the seizure onset zone in the brain. The second operation cuts out the onset zone.
“Our goal is to one day not need invasive monitoring,” said Sandipan Pati, M.D., assistant professor of neurology. “That would mean one surgery instead of two, and the patient would not have to stay in the hospital for two weeks.”
The UAB team in the epileptic brain seizure study — headed by Jerzy P. Szaflarski, M.D., Ph.D., professor of neurology — is developing and validating software for noninvasive brain mapping that will allow caregivers to locate that part of the brain that initiates seizures and locate those parts that function in memory.
“We will provide a road map for the surgeons — where to operate to remove the thumb-sized part of the brain that kicks off seizures, and what parts of the brain to avoid,” Pati said, “so that patients will have no added memory deficits after the operation.”
The investigators will use magnetoencephalography, or MEG, to map electrical activity using the magnetic fields produced by natural electrical currents produced by the brain. The magnetic forces are measured from the outside of the brain as the top of a patient’s head fits into the MEG device, which looks something like a beauty-shop hair drier on steroids. Pati says the UAB team has preliminary data about using the MEG to identify the onset zone by its hyper-excitation, without the need to wait for seizures.
Other UAB investigators in the study are Roy Martin, Ph.D., associate professor of neurology, and Timothy Gawne, Ph.D., associate professor of vision sciences. The lead institution for the study is Louisiana Tech University, and the University of Arkansas is also a partner in the study.
 From left: Mark Bolding, Lynn Dobrunz, Lori McMahon and Gary Gray.Optogenetics uses light to control cells in living tissue, after light-sensitive ion channels are introduced into the cells by gene manipulation. Then light is sent into the brain on a fiber-optic cable inserted into the brain, to make neurons fire or to stop neurons from firing. This control helps researchers learn how the brain is wired and how it works.
From left: Mark Bolding, Lynn Dobrunz, Lori McMahon and Gary Gray.Optogenetics uses light to control cells in living tissue, after light-sensitive ion channels are introduced into the cells by gene manipulation. Then light is sent into the brain on a fiber-optic cable inserted into the brain, to make neurons fire or to stop neurons from firing. This control helps researchers learn how the brain is wired and how it works.
The optogenetics project will create technology to control the light-sensitive ion channels using low-power X-rays, thus allowing control of neurons from outside the body.
McMahon is the UAB co-principal investigator in this project, which is led by Clemson University. Other partner institutions are the University of New Mexico and the University of South Carolina. The Clemson researchers will develop special nanoparticles that emit light in response to X-rays, the New Mexico researchers will genetically modify the light-sensitive ion channels so that they can bind the nanoparticles, and the UAB researchers will test how those nanoparticles disperse in the brain and how these particles, when activated by X-rays, can turn on and off brain circuits.
“We are about to do the first validation,” McMahon said. “The entire four years of the grant is developing the tool. Then we can use it in animal disease models for Alzheimer’s disease, Parkinson’s disease and anxiety. The goal is to understand brain circuitry using preclinical models of neurologic disease and models of neuropsychiatric illness.”
Other UAB investigators are Lynn Dobrunz, Ph.D., associate professor of neurobiology; Mark Bolding, Ph.D., assistant professor in the Division of Advanced Medical Imaging Research, Department of Radiology, and director of the Civitan International Neuroimaging Facility; Kazutoshi Nakazawa, M.D., Ph.D., associate professor of psychiatry and behavioral neurobiology; and Gary Gray, Ph.D., professor of chemistry.
 Jacques Wadiche, Farah Lubin and Paul Gamlin.It has long been known that neural activity is associated with increased blood flow, as the neurons need more oxygen and nutrients. In imaging with functional magnetic resonance, or fMRI, increased blood flow can be seen in parts of the brain as the subjects perform a task. But the resolution — both spatially and in time — is not sharp, and fMRI remains an indirect measure of neural activity.
Jacques Wadiche, Farah Lubin and Paul Gamlin.It has long been known that neural activity is associated with increased blood flow, as the neurons need more oxygen and nutrients. In imaging with functional magnetic resonance, or fMRI, increased blood flow can be seen in parts of the brain as the subjects perform a task. But the resolution — both spatially and in time — is not sharp, and fMRI remains an indirect measure of neural activity.
The UAB team will use a very expensive infrared laser and microscope to peer beneath the surface of living brains to look at individual neurons and capillary beds.
“We will image cells in the brain using two-photon imaging,” said Paul Gamlin, Ph.D., professor of ophthalmology and UAB’s co-principal investigator in the neural activity and blood flow effort. “While monitoring neural activity, we will also monitor what the capillary bed is doing.”
“The question is, if we stimulate the cells, how precise is the blood flow change?”
Capillaries are the smallest blood vessels, bringing oxygen and nutrients to cells and taking away carbon dioxide and waste products. Networks of capillaries form tiny beds of vessels, and each capillary has a precapillary sphincter, a ring of muscle that can control blood flow, akin to crimping a garden hose to lessen water flow. It has been estimated that the human brain has 400 miles of capillaries, and that nearly every neuron in the brain has its own capillary.
Gamlin and others on the UAB team — Lawrence Sincich, Ph.D., assistant professor of vision sciences; Farah Lubin, Ph.D., associate professor of neurobiology; Jacques Wadiche, Ph.D., associate professor of neurobiology; and Yuhua Zhang, Ph.D., assistant professor of ophthalmology — can stimulate neurons nonphysiologically and see how the capillaries change. They will also look at physiological stimulation by shining light on one, two or three retinal cells in the eye and watching how neurons and capillaries in the visual cortex of the brain respond.
The lead institution in this project is the Medical University of South Carolina, and Furman University and the University of South Carolina, Beaufort, are partners in the study.
 Eugenia KharlampievaThe Gulf Coast aquatic ecosystem hosts important fishing grounds and aquaculture, which co-exist with trading ports, off-shore oil wells and production industries. The fourth UAB EPSCoR is aimed at monitoring the water quality of this ecosystem, under the lead of the University of Southern Mississippi.
Eugenia KharlampievaThe Gulf Coast aquatic ecosystem hosts important fishing grounds and aquaculture, which co-exist with trading ports, off-shore oil wells and production industries. The fourth UAB EPSCoR is aimed at monitoring the water quality of this ecosystem, under the lead of the University of Southern Mississippi.
Ten researchers at six institutions in Alabama and Mississippi will develop advanced polymer-based, selective sensing technologies to detect and analyze pollutants.
The UAB investigator is Eugenia Kharlampieva, Ph.D., associate professor of polymer chemistry. She will design and synthesize three-dimensional porous hydrogel microparticles. These particles will be filled with sensing molecules created by Marco Bonizzoni, assistant professor of chemistry at the University of Alabama, Tuscaloosa. These hydrogels will eventually become devices that can sense polycyclic aromatic hydrocarbons in sea water.
“Sea water is a challenge because it is very rich in ions from all that salt,” Kharlampieva said. “It is hard to find the right technology that will work in that environment.”
Other researchers in the grant are developing sensitive and selective sensors to measure levels of carbon dioxide, nitrates and phosphates. The goal is new classes of seawater quality sensors that are faster, simpler and less costly. Other partner institutions in the grant are the University of Mississippi, Mississippi State University and Jackson State University.
Alabama now has more EPSCoR Track II grants than any other state following the award of basic science grants meant to stimulate competitive research in regions of the country traditionally less able to compete for such research funds.
Four teams of University of Alabama at Birmingham researchers have been awarded National Science Foundation grants totaling $5.4 million meant to stimulate competitive research in regions of the country that are less able to compete for these research funds.
One research team supported by an NSF grant is in the College of Arts and Sciences’ Department of Chemistry, led by a polymer chemist who applies nanotechnology to biological and biomedical challenges. The three other grants will support basic neuroscience studies, one of UAB’s hallmark research strengths.
Lori McMahon, Ph.D., the Jarman F. Lowder Professor of Neuroscience, dean of the UAB Graduate School and director of the UAB Comprehensive Neuroscience Center, highlighted the three neuroscience EPSCoR grants at this fall’s Comprehensive Neuroscience Center retreat, calling them prestigious and competitive.
“UAB neuroscience has never had one NSF grant, and now we have three,” she said. “With the results of these grants, we can increase our funding beyond the National Institutes of Health.”
These four UAB grants, and one additional Alabama-related EPSCoR that supports research teams at the University of Alabama, Tuscaloosa, and the University of Mississippi, set a record, says Christopher Lawson, Ph.D., executive director of the Alabama EPSCoR program and a professor in the UAB Department of Physics.
“The state of Alabama now has more of the EPSCoR Track II grants than any other state; no state has ever had five.”
Only 25 states, two territories and one commonwealth — areas that receive much less NSF funding than the major research universities in the other 25 states — qualify to compete for the Experimental Program to Stimulate Competitive Research grants, known as EPSCoR. The EPSCoR Track II grants are meant to level the playing fields among have and have-not research states, and applicants must form collaborations with researchers from other EPSCoR states. This means the UAB teams have formed synergistic partnerships and collaborations across the Birmingham campus and with scientists in other states to foster regional research strength.

UAB neuroscience teams and their goals
The three neuroscience EPSCoR grants support a study to understand the initiation of epileptic brain seizures; a project to develop a new tool for optogenetics, which is the control of neural cells using light; and an effort to discover a universal rule for the relation between neural activity and increased blood flow in areas of the brain. In all three EPSCoR grants, UAB is a partner institution, and the lead institution is in another EPSCoR state.“Several institutions reached out to UAB because we are so strong in neuroscience,” McMahon said. “There is a lot of UAB synergy around the three neuroscience EPSCoRs.”
McMahon says all three UAB neuroscience teams will meet regularly to share results and ideas.
Epileptic brain seizures
 From left: Roy Martin, Jerzy P. Szaflarski and Timothy Gawne.The current approach for epilepsy surgery at UAB involves two surgeries. The first implants electrodes into a patient’s brain for a two-week period to map the location of the seizure onset zone in the brain. The second operation cuts out the onset zone.
From left: Roy Martin, Jerzy P. Szaflarski and Timothy Gawne.The current approach for epilepsy surgery at UAB involves two surgeries. The first implants electrodes into a patient’s brain for a two-week period to map the location of the seizure onset zone in the brain. The second operation cuts out the onset zone. “Our goal is to one day not need invasive monitoring,” said Sandipan Pati, M.D., assistant professor of neurology. “That would mean one surgery instead of two, and the patient would not have to stay in the hospital for two weeks.”
The UAB team in the epileptic brain seizure study — headed by Jerzy P. Szaflarski, M.D., Ph.D., professor of neurology — is developing and validating software for noninvasive brain mapping that will allow caregivers to locate that part of the brain that initiates seizures and locate those parts that function in memory.
“We will provide a road map for the surgeons — where to operate to remove the thumb-sized part of the brain that kicks off seizures, and what parts of the brain to avoid,” Pati said, “so that patients will have no added memory deficits after the operation.”
The investigators will use magnetoencephalography, or MEG, to map electrical activity using the magnetic fields produced by natural electrical currents produced by the brain. The magnetic forces are measured from the outside of the brain as the top of a patient’s head fits into the MEG device, which looks something like a beauty-shop hair drier on steroids. Pati says the UAB team has preliminary data about using the MEG to identify the onset zone by its hyper-excitation, without the need to wait for seizures.
Other UAB investigators in the study are Roy Martin, Ph.D., associate professor of neurology, and Timothy Gawne, Ph.D., associate professor of vision sciences. The lead institution for the study is Louisiana Tech University, and the University of Arkansas is also a partner in the study.
New tool for optogenetics
 From left: Mark Bolding, Lynn Dobrunz, Lori McMahon and Gary Gray.Optogenetics uses light to control cells in living tissue, after light-sensitive ion channels are introduced into the cells by gene manipulation. Then light is sent into the brain on a fiber-optic cable inserted into the brain, to make neurons fire or to stop neurons from firing. This control helps researchers learn how the brain is wired and how it works.
From left: Mark Bolding, Lynn Dobrunz, Lori McMahon and Gary Gray.Optogenetics uses light to control cells in living tissue, after light-sensitive ion channels are introduced into the cells by gene manipulation. Then light is sent into the brain on a fiber-optic cable inserted into the brain, to make neurons fire or to stop neurons from firing. This control helps researchers learn how the brain is wired and how it works.The optogenetics project will create technology to control the light-sensitive ion channels using low-power X-rays, thus allowing control of neurons from outside the body.
McMahon is the UAB co-principal investigator in this project, which is led by Clemson University. Other partner institutions are the University of New Mexico and the University of South Carolina. The Clemson researchers will develop special nanoparticles that emit light in response to X-rays, the New Mexico researchers will genetically modify the light-sensitive ion channels so that they can bind the nanoparticles, and the UAB researchers will test how those nanoparticles disperse in the brain and how these particles, when activated by X-rays, can turn on and off brain circuits.
“We are about to do the first validation,” McMahon said. “The entire four years of the grant is developing the tool. Then we can use it in animal disease models for Alzheimer’s disease, Parkinson’s disease and anxiety. The goal is to understand brain circuitry using preclinical models of neurologic disease and models of neuropsychiatric illness.”
Other UAB investigators are Lynn Dobrunz, Ph.D., associate professor of neurobiology; Mark Bolding, Ph.D., assistant professor in the Division of Advanced Medical Imaging Research, Department of Radiology, and director of the Civitan International Neuroimaging Facility; Kazutoshi Nakazawa, M.D., Ph.D., associate professor of psychiatry and behavioral neurobiology; and Gary Gray, Ph.D., professor of chemistry.
Neural activity and blood flow
 Jacques Wadiche, Farah Lubin and Paul Gamlin.It has long been known that neural activity is associated with increased blood flow, as the neurons need more oxygen and nutrients. In imaging with functional magnetic resonance, or fMRI, increased blood flow can be seen in parts of the brain as the subjects perform a task. But the resolution — both spatially and in time — is not sharp, and fMRI remains an indirect measure of neural activity.
Jacques Wadiche, Farah Lubin and Paul Gamlin.It has long been known that neural activity is associated with increased blood flow, as the neurons need more oxygen and nutrients. In imaging with functional magnetic resonance, or fMRI, increased blood flow can be seen in parts of the brain as the subjects perform a task. But the resolution — both spatially and in time — is not sharp, and fMRI remains an indirect measure of neural activity.The UAB team will use a very expensive infrared laser and microscope to peer beneath the surface of living brains to look at individual neurons and capillary beds.
“We will image cells in the brain using two-photon imaging,” said Paul Gamlin, Ph.D., professor of ophthalmology and UAB’s co-principal investigator in the neural activity and blood flow effort. “While monitoring neural activity, we will also monitor what the capillary bed is doing.”
“The question is, if we stimulate the cells, how precise is the blood flow change?”
Capillaries are the smallest blood vessels, bringing oxygen and nutrients to cells and taking away carbon dioxide and waste products. Networks of capillaries form tiny beds of vessels, and each capillary has a precapillary sphincter, a ring of muscle that can control blood flow, akin to crimping a garden hose to lessen water flow. It has been estimated that the human brain has 400 miles of capillaries, and that nearly every neuron in the brain has its own capillary.
Gamlin and others on the UAB team — Lawrence Sincich, Ph.D., assistant professor of vision sciences; Farah Lubin, Ph.D., associate professor of neurobiology; Jacques Wadiche, Ph.D., associate professor of neurobiology; and Yuhua Zhang, Ph.D., assistant professor of ophthalmology — can stimulate neurons nonphysiologically and see how the capillaries change. They will also look at physiological stimulation by shining light on one, two or three retinal cells in the eye and watching how neurons and capillaries in the visual cortex of the brain respond.
The lead institution in this project is the Medical University of South Carolina, and Furman University and the University of South Carolina, Beaufort, are partners in the study.
Detecting pollutants in Gulf Coast marine ecosystems
 Eugenia KharlampievaThe Gulf Coast aquatic ecosystem hosts important fishing grounds and aquaculture, which co-exist with trading ports, off-shore oil wells and production industries. The fourth UAB EPSCoR is aimed at monitoring the water quality of this ecosystem, under the lead of the University of Southern Mississippi.
Eugenia KharlampievaThe Gulf Coast aquatic ecosystem hosts important fishing grounds and aquaculture, which co-exist with trading ports, off-shore oil wells and production industries. The fourth UAB EPSCoR is aimed at monitoring the water quality of this ecosystem, under the lead of the University of Southern Mississippi.Ten researchers at six institutions in Alabama and Mississippi will develop advanced polymer-based, selective sensing technologies to detect and analyze pollutants.
The UAB investigator is Eugenia Kharlampieva, Ph.D., associate professor of polymer chemistry. She will design and synthesize three-dimensional porous hydrogel microparticles. These particles will be filled with sensing molecules created by Marco Bonizzoni, assistant professor of chemistry at the University of Alabama, Tuscaloosa. These hydrogels will eventually become devices that can sense polycyclic aromatic hydrocarbons in sea water.
“Sea water is a challenge because it is very rich in ions from all that salt,” Kharlampieva said. “It is hard to find the right technology that will work in that environment.”
Other researchers in the grant are developing sensitive and selective sensors to measure levels of carbon dioxide, nitrates and phosphates. The goal is new classes of seawater quality sensors that are faster, simpler and less costly. Other partner institutions in the grant are the University of Mississippi, Mississippi State University and Jackson State University.
Molecular Scissors.....
By: Vicki Hixon
Published Date: Dec 07
Molecular scissors could point the way to genetic cures
By Erin Burns, Amber Guidry, Nicholas Potochick, and Charles Buchanan • Photos by Steve Wood
Guan-En Graham is determined to find out exactly what happened to her father. When she was a child, he developed brain cancer. Since then, she has worked to understand the intricate genetic mechanisms that trigger brain diseases so that one day, perhaps, she can shut them down for good.
Now Graham might have the tool to do it. In the lab of Jeremy Day, Ph.D., the sophomore neuroscience major from Las Vegas is part of a UAB research team investigating CRISPR, a piece of gene-editing technology that could have the potential to prevent disease before patients start to suffer.
 Jeremy Day (center) and undergraduate students Guan-En Graham (left) and Jasmin Revanna (right) use CRISPR technology to illuminate the power and reach of epigenetic modifications in brain diseases.
Jeremy Day (center) and undergraduate students Guan-En Graham (left) and Jasmin Revanna (right) use CRISPR technology to illuminate the power and reach of epigenetic modifications in brain diseases.
The concept of adding, deleting, or otherwise altering an existing DNA sequence is nothing new; scientists have done it in laboratories for decades. But earlier gene-editing technologies can produce “off-target” effects, unintentionally tinkering with other pieces of DNA. CRISPR is more precise, efficient, versatile, and affordable than its predecessors, which has changed how scientists address questions and the speed in which they answer them.
Day, a UAB School of Medicine assistant professor of neurobiology, studies epigenetics, a group of molecular modifications that influence gene activity without changing the DNA sequence. CRISPR enables his research group “to explore how epigenetic modifications affect expression of specific genes. The ability to express genes in a selective fashion gives rise to the amazing diversity of cell types that we possess.”
Jasmin Revanna, another neuroscience major working with Day, describes the work as “changing ‘tags’ on DNA that affect its function in the cell.” The goal is to discover the roles those tags, or epigenetic modifications, play in diseases that don’t result from DNA mutations, adds the freshman from Jacksonville, Alabama.
“We are applying this to addiction-related diseases to understand how drugs of abuse engage the epigenome to generate long-term alterations in neuron function and behavior,” Day says. The findings could lay the groundwork for a variety of CRISPR-related epigenetic treatments—perhaps boosting or restoring memories for Alzheimer’s patients or tamping them down for people with post-traumatic stress disorder. “While our current studies are in their infancy, this technology is a huge leap forward,” Day says.

Where CRISPR cuts DNA, researchers can delete or insert a DNA sequence. Townes’s lab has applied this technology to successfully correct the genetic mutation causing sickle cell disease (SCD), which can cause chronic pain and organ damage. Current SCD treatments are lifelong and can have severe side effects.
“All patients with SCD have the exact same mutation in the exact same location on the exact same gene—meaning we need to correct only one mutation in the bone marrow cells to cure someone of this condition forever,” Townes says. The process involves retrieving bone marrow cells from the patient, treating the genes, and then implanting the cells back into the patient. Because the cells are the patient’s own, there would be no need for a transplant or risk of rejection. “With CRISPR we have an effective rate of correction for the sickle cell mutation between 30 and 50 percent. Just that portion of cells is enough to cure someone of sickle cell,” Townes says.
Currently, each baby born in the United States is tested for the sickle mutation, allowing researchers to know within a month whether the baby will develop the disease. SCD symptoms typically do not appear until children are a year old, “which leaves 11 months to cure them, and they may never have to experience the disease,” Townes says. A similar approach could benefit patients with other diseases. In published research, Townes and his team have shown how they used CRISPR to correct the DNA that triggers severe combined immunodeficiency disease, in which children are born with no immune response.
 Researchers led by Tim Townes (left) have used CRISPR (illustrated above) to correct the genetic mutation causing sickle cell disease—a discovery that points to a potential cure.
Researchers led by Tim Townes (left) have used CRISPR (illustrated above) to correct the genetic mutation causing sickle cell disease—a discovery that points to a potential cure.
Babies born in Alabama hospitals currently are tested for 35 diseases, “but for about the same price, we could sequence their genome” and pinpoint genes that put them at risk for disease, Townes says. Then, “long before someone develops one of these genetic predispositions, we could correct the gene so that they never experience that disease,” Townes says.
Even when gene editing is difficult or impossible, CRISPR’s ability to introduce desirable epigenetic modifications may become highly useful, Day adds. "This will be possible in several years as we develop our basic understanding of how epigenetic marks regulate single genes."
Townes agrees, noting that “overall the scientific community has done well in regulating these new technologies and not acting irresponsibly.”
The Food and Drug Administration, which must approve any clinical trial of CRISPR in humans, also is developing regulations on safety and the use of gene-editing technology.
• Learn more about the groundbreaking research and educational opportunities in the UAB Department of Neurobiology and Department of Biochemistry and Molecular Genetics.
• Give something and change everything for School of Medicine researchers seeking cures for diseases and the patients who could benefit from them.
back to top
By Erin Burns, Amber Guidry, Nicholas Potochick, and Charles Buchanan • Photos by Steve Wood
Guan-En Graham is determined to find out exactly what happened to her father. When she was a child, he developed brain cancer. Since then, she has worked to understand the intricate genetic mechanisms that trigger brain diseases so that one day, perhaps, she can shut them down for good.
Now Graham might have the tool to do it. In the lab of Jeremy Day, Ph.D., the sophomore neuroscience major from Las Vegas is part of a UAB research team investigating CRISPR, a piece of gene-editing technology that could have the potential to prevent disease before patients start to suffer.
Precise and Programmable
CRISPR, or Clustered Regularly Interspaced Short Palindromic Repeats, is essentially a pair of molecule-sized programmable scissors—scissors that work on the DNA inside living cells. In the few years since scientists have refined the technology, CRISPR has revolutionized gene editing. Jeremy Day (center) and undergraduate students Guan-En Graham (left) and Jasmin Revanna (right) use CRISPR technology to illuminate the power and reach of epigenetic modifications in brain diseases.
Jeremy Day (center) and undergraduate students Guan-En Graham (left) and Jasmin Revanna (right) use CRISPR technology to illuminate the power and reach of epigenetic modifications in brain diseases.The concept of adding, deleting, or otherwise altering an existing DNA sequence is nothing new; scientists have done it in laboratories for decades. But earlier gene-editing technologies can produce “off-target” effects, unintentionally tinkering with other pieces of DNA. CRISPR is more precise, efficient, versatile, and affordable than its predecessors, which has changed how scientists address questions and the speed in which they answer them.
Day, a UAB School of Medicine assistant professor of neurobiology, studies epigenetics, a group of molecular modifications that influence gene activity without changing the DNA sequence. CRISPR enables his research group “to explore how epigenetic modifications affect expression of specific genes. The ability to express genes in a selective fashion gives rise to the amazing diversity of cell types that we possess.”
Jasmin Revanna, another neuroscience major working with Day, describes the work as “changing ‘tags’ on DNA that affect its function in the cell.” The goal is to discover the roles those tags, or epigenetic modifications, play in diseases that don’t result from DNA mutations, adds the freshman from Jacksonville, Alabama.
“We are applying this to addiction-related diseases to understand how drugs of abuse engage the epigenome to generate long-term alterations in neuron function and behavior,” Day says. The findings could lay the groundwork for a variety of CRISPR-related epigenetic treatments—perhaps boosting or restoring memories for Alzheimer’s patients or tamping them down for people with post-traumatic stress disorder. “While our current studies are in their infancy, this technology is a huge leap forward,” Day says.

Cut, Paste, Treat
CRISPR originated in certain bacteria, where it functions as protection against invading viruses. Essentially, it’s a “highly efficient and specific” cellular immune system, explains Tim Townes, Ph.D., professor of biochemistry and molecular genetics in the UAB School of Medicine. The molecular mechanism recognizes viral DNA and cuts it up to prevent the virus from replicating inside the bacteria.Where CRISPR cuts DNA, researchers can delete or insert a DNA sequence. Townes’s lab has applied this technology to successfully correct the genetic mutation causing sickle cell disease (SCD), which can cause chronic pain and organ damage. Current SCD treatments are lifelong and can have severe side effects.
“All patients with SCD have the exact same mutation in the exact same location on the exact same gene—meaning we need to correct only one mutation in the bone marrow cells to cure someone of this condition forever,” Townes says. The process involves retrieving bone marrow cells from the patient, treating the genes, and then implanting the cells back into the patient. Because the cells are the patient’s own, there would be no need for a transplant or risk of rejection. “With CRISPR we have an effective rate of correction for the sickle cell mutation between 30 and 50 percent. Just that portion of cells is enough to cure someone of sickle cell,” Townes says.
Currently, each baby born in the United States is tested for the sickle mutation, allowing researchers to know within a month whether the baby will develop the disease. SCD symptoms typically do not appear until children are a year old, “which leaves 11 months to cure them, and they may never have to experience the disease,” Townes says. A similar approach could benefit patients with other diseases. In published research, Townes and his team have shown how they used CRISPR to correct the DNA that triggers severe combined immunodeficiency disease, in which children are born with no immune response.
A Key to Personalized Medicine
Both Townes and Day expect human clinical trials of CRISPR-based therapeutics to begin in the next few years. Genetic disorders arising from single-gene mutations, like SCD, are ideal CRISPR candidates because they require only one small correction to be made, and the correction is always the same. However, most genetic disorders aren’t so simple, involving complex mutations in multiple genes. As CRISPR technology improves, it may be adaptable for a wider range of disorders, and both Townes and Day predict that it will play a key role in the development of personalized medicine, with treatments tailored to each patient’s genetic makeup. Researchers led by Tim Townes (left) have used CRISPR (illustrated above) to correct the genetic mutation causing sickle cell disease—a discovery that points to a potential cure.
Researchers led by Tim Townes (left) have used CRISPR (illustrated above) to correct the genetic mutation causing sickle cell disease—a discovery that points to a potential cure.Babies born in Alabama hospitals currently are tested for 35 diseases, “but for about the same price, we could sequence their genome” and pinpoint genes that put them at risk for disease, Townes says. Then, “long before someone develops one of these genetic predispositions, we could correct the gene so that they never experience that disease,” Townes says.
Even when gene editing is difficult or impossible, CRISPR’s ability to introduce desirable epigenetic modifications may become highly useful, Day adds. "This will be possible in several years as we develop our basic understanding of how epigenetic marks regulate single genes."
Safety Monitors
Could CRISPR lead science down a slippery slope, with people wanting to alter their genes—or their children’s genes—for cosmetic reasons, for example? Safety and ethical considerations are key to CRISPR’s progress, Day and Townes say. “We already use many different therapeutics that are capable of altering how our genes are expressed and capable of producing long-term effects,” Day says. “The ethical considerations that arise have more to do with the application of technology than with the technology itself. Ultimately, the benefits of CRISPR will outweigh potential risks, provided there is adequate screening and careful observation.”Townes agrees, noting that “overall the scientific community has done well in regulating these new technologies and not acting irresponsibly.”
The Food and Drug Administration, which must approve any clinical trial of CRISPR in humans, also is developing regulations on safety and the use of gene-editing technology.
• Learn more about the groundbreaking research and educational opportunities in the UAB Department of Neurobiology and Department of Biochemistry and Molecular Genetics.
• Give something and change everything for School of Medicine researchers seeking cures for diseases and the patients who could benefit from them.
Published October 2016
back to top
McKnight Poster Winners 2016
By: Vicki Hixon
Published Date: Nov 16
Congratulations to the following for their outstanding abstracts and poster presentations at the McKnight Poster Session held on Sunday, November 13, 2016 in San Diego, California. It was very competitive this year, with a record 67 poster presenters from the various McKnight Institutes.
First place winner was Joseph McQuail from the University of Florida, for his presentation of “Stress reactivity predicts impaired working memory in aging: vulnerability of GABAergic synapses.”
Representing the University of Arizona, Rachel Samson was awarded second place with “Expectation of large reward elicits bursts of beta-band oscillations in the aged rat amygdala.”
Third place winner was Natalie Khoury from the University of Miami, for presenting “Elucidating the molecular mechanism behind the long-term cerebral ischemic tolerance mediated by resveratrol preconditioning.”
Join us in congratulating the winners for their outstanding work!
First place winner was Joseph McQuail from the University of Florida, for his presentation of “Stress reactivity predicts impaired working memory in aging: vulnerability of GABAergic synapses.”
Representing the University of Arizona, Rachel Samson was awarded second place with “Expectation of large reward elicits bursts of beta-band oscillations in the aged rat amygdala.”
Third place winner was Natalie Khoury from the University of Miami, for presenting “Elucidating the molecular mechanism behind the long-term cerebral ischemic tolerance mediated by resveratrol preconditioning.”
Join us in congratulating the winners for their outstanding work!
Hike for Julie
McKnight Poster Reception - Abstracts - November 13, 2016
Neuroscience trainees navigate challenges beyond the lab
By: Vicki Hixon
Published Date: Oct 14
By Nancy Mann Jackson • Photos by Steve Wood
Even though she possessed a bachelor’s degree in chemistry and a master’s in biotechnology, Lillian Brady felt that she didn’t fit into the booming field of neuroscience. “As an underrepresented student, it can be easy to get into the mind frame that you don’t belong,” says Brady, a graduate of Alcorn State University, a historically black university in southwest Mississippi.
 Each Roadmap Scholar works closely with a research mentor. Here, scholar Lillian Brady (right) meets with neurobiology associate professor Lynn Dobrunz.
Each Roadmap Scholar works closely with a research mentor. Here, scholar Lillian Brady (right) meets with neurobiology associate professor Lynn Dobrunz.
But then the Jackson, Mississippi, native found a place where she fit in perfectly: UAB’s Neuroscience Roadmap Scholars program, which is designed to help engage and retain underrepresented graduate trainees—including ethnic minorities and students with disabilities—in the neuroscience workforce.
Brady, now a doctoral student in UAB’s Department of Neurobiology, calls the program a “confidence booster” that has provided both support and encouragement. “I’ve been exposed to scientists who look like me and who have had some of the same challenges I am facing now,” she says. “These scientists are thriving in their fields, so I have no doubt that I can be successful in whatever career path I choose.”
The problem isn’t talent or academic strength; rather, many students from diverse backgrounds or with disabilities may struggle with financial challenges, family needs, or a lack of confidence. Lubin and Lori McMahon, Ph.D., UAB Graduate School dean and UAB Comprehensive Neuroscience Center director, have seen such roadblocks dismantle the plans and goals of numerous students and coworkers.
 Roadmap co-directors Lori McMahon (left) and Farah Lubin (right) serve as career coaches for scholars outside the lab.
Roadmap co-directors Lori McMahon (left) and Farah Lubin (right) serve as career coaches for scholars outside the lab.
To plug the pipeline’s holes, Lubin and McMahon developed Roadmap Scholars to emphasize mentoring, support networks, team-building experiences, and a community of peers to help guide more students to achieve neuroscience careers. Funded by a grant from the National Institute of Neurological Disorders and Stroke, the program launched in 2014. Today it includes 25 doctoral students.
The funding does not pay tuition or stipends; instead, it enhances and provides educational experiences to prepare students for further study and to encourage them to remain in neuroscience. And it works in tandem with the students’ graduate curriculum. For example, every Roadmap Scholar matches with a “career coach” in addition to a primary research mentor. The coach is a faculty member not on the student’s thesis committee who is available to talk about nearly anything—scientific projects, publishing and presenting, conflict management, or life in general, Lubin says. In doing so, these coaches provide additional professional perspectives and serve as an additional layer of support.
For Leland Fleming, a Fort Worth, Texas, native and UAB Graduate Biomedical Sciences doctoral student, the program’s peer and faculty network has made his goal of becoming a neuroscientist more tangible. “With its guidance and inspiration, it has made a once seemingly impossible goal something that I feel more than capable of accomplishing,” he says.
 Leland Fleming (right) with his research mentor, neurobiology assistant professor Kristina Visscher
Leland Fleming (right) with his research mentor, neurobiology assistant professor Kristina Visscher
For Fleming, key conference takeaways included insights on common challenges that minority students can face in graduate school. These might be “feelings of inadequacy or feelings that he or she is merely an impostor on the brink of being exposed at any given moment,” he explains. But he and other students also received “terrific advice on coping with these issues when they arise,” Fleming says.
 Lubin serves as a research mentor for scholar Rylie Hightower (right).
Lubin serves as a research mentor for scholar Rylie Hightower (right).
The conference also helps to build strong bonds among students. Matthew Timberlake, a second-year graduate student who grew up in Fort Worth and Enterprise, Alabama, has enjoyed helping his colleagues to design and present lectures—even sharing his own research data with them. “I like the sense of community,” he says. “My colleagues and I—especially the upperclassmen—have an important role in discussions,” he says. “We help to ease some of the unknown for the underclassmen. In the same way, I also benefit from the upperclassmen’s advice and guidance.”
Rylie Hightower, an Albuquerque native who came to UAB after earning a nursing degree in New Mexico, is one of the newer students benefiting from those discussions. At a Roadmap spring retreat, Hightower sought advice from older students. Those interactions “will help me throughout my time at UAB and beyond,” she says.
The program has “made me feel at home away from home,” Hightower says. “But it also has helped me understand that a supportive community can greatly contribute to the way I think and perform as a student and as a scientist.”
 Matthew Timberlake works with research mentor and psychiatry professor Yogesh Dwivedi.
Matthew Timberlake works with research mentor and psychiatry professor Yogesh Dwivedi.
Every student also learns about neuroscience-related job opportunities in business and industry. “Often, students are encouraged to build a career path focused on remaining in academia,” says Megan Rich, a Fairfield, Connecticut, native and UAB Graduate Biomedical Sciences student. “The program has been great about expressing other options after graduate school and that it is OK to think about them.”
By helping these students to feel empowered, capable, and supported, the Neuroscience Roadmap Scholars program will lay the groundwork for future progress in one of the fastest growing scientific fields, McMahon says. Lubin agrees, noting that diversity is crucial for continuing advancements: “Diverse groups can offer unique perspectives and unique ideas, which will push the field forward.”
• Learn more about the Neuroscience Roadmap Scholars Program, including how to apply.
• Give something and change everything for the next generation of neuroscientists by supporting the School of Medicine.
Even though she possessed a bachelor’s degree in chemistry and a master’s in biotechnology, Lillian Brady felt that she didn’t fit into the booming field of neuroscience. “As an underrepresented student, it can be easy to get into the mind frame that you don’t belong,” says Brady, a graduate of Alcorn State University, a historically black university in southwest Mississippi.
 Each Roadmap Scholar works closely with a research mentor. Here, scholar Lillian Brady (right) meets with neurobiology associate professor Lynn Dobrunz.
Each Roadmap Scholar works closely with a research mentor. Here, scholar Lillian Brady (right) meets with neurobiology associate professor Lynn Dobrunz.But then the Jackson, Mississippi, native found a place where she fit in perfectly: UAB’s Neuroscience Roadmap Scholars program, which is designed to help engage and retain underrepresented graduate trainees—including ethnic minorities and students with disabilities—in the neuroscience workforce.
Brady, now a doctoral student in UAB’s Department of Neurobiology, calls the program a “confidence booster” that has provided both support and encouragement. “I’ve been exposed to scientists who look like me and who have had some of the same challenges I am facing now,” she says. “These scientists are thriving in their fields, so I have no doubt that I can be successful in whatever career path I choose.”
Strengthening the Pipeline
“Through our experience working with students, we realized the pipeline for diverse neuroscientists was leaky,” recalls Farah Lubin, Ph.D., associate professor of neurobiology. “We might start with many diverse, motivated students, but somehow, many of them don’t make it into successful careers.”The problem isn’t talent or academic strength; rather, many students from diverse backgrounds or with disabilities may struggle with financial challenges, family needs, or a lack of confidence. Lubin and Lori McMahon, Ph.D., UAB Graduate School dean and UAB Comprehensive Neuroscience Center director, have seen such roadblocks dismantle the plans and goals of numerous students and coworkers.
 Roadmap co-directors Lori McMahon (left) and Farah Lubin (right) serve as career coaches for scholars outside the lab.
Roadmap co-directors Lori McMahon (left) and Farah Lubin (right) serve as career coaches for scholars outside the lab.To plug the pipeline’s holes, Lubin and McMahon developed Roadmap Scholars to emphasize mentoring, support networks, team-building experiences, and a community of peers to help guide more students to achieve neuroscience careers. Funded by a grant from the National Institute of Neurological Disorders and Stroke, the program launched in 2014. Today it includes 25 doctoral students.
The funding does not pay tuition or stipends; instead, it enhances and provides educational experiences to prepare students for further study and to encourage them to remain in neuroscience. And it works in tandem with the students’ graduate curriculum. For example, every Roadmap Scholar matches with a “career coach” in addition to a primary research mentor. The coach is a faculty member not on the student’s thesis committee who is available to talk about nearly anything—scientific projects, publishing and presenting, conflict management, or life in general, Lubin says. In doing so, these coaches provide additional professional perspectives and serve as an additional layer of support.
For Leland Fleming, a Fort Worth, Texas, native and UAB Graduate Biomedical Sciences doctoral student, the program’s peer and faculty network has made his goal of becoming a neuroscientist more tangible. “With its guidance and inspiration, it has made a once seemingly impossible goal something that I feel more than capable of accomplishing,” he says.
 Leland Fleming (right) with his research mentor, neurobiology assistant professor Kristina Visscher
Leland Fleming (right) with his research mentor, neurobiology assistant professor Kristina VisscherCreating a Community
The support system kicks into gear even before the scholars’ first semester at the program’s summer NEURAL (National Enhancement of Underrepresented Academic Leaders) Conference, which draws neuroscience trainees from across the country. There, students can hone their networking and public speaking skills and interact with the field’s academic leaders. For instance, at the 2015 inaugural conference, leading neuroscientist Roger Nicoll, M.D., of the University of California San Francisco drew an emotional response from the audience when he spoke of succeeding despite his lifelong struggle with dyslexia.For Fleming, key conference takeaways included insights on common challenges that minority students can face in graduate school. These might be “feelings of inadequacy or feelings that he or she is merely an impostor on the brink of being exposed at any given moment,” he explains. But he and other students also received “terrific advice on coping with these issues when they arise,” Fleming says.
 Lubin serves as a research mentor for scholar Rylie Hightower (right).
Lubin serves as a research mentor for scholar Rylie Hightower (right).The conference also helps to build strong bonds among students. Matthew Timberlake, a second-year graduate student who grew up in Fort Worth and Enterprise, Alabama, has enjoyed helping his colleagues to design and present lectures—even sharing his own research data with them. “I like the sense of community,” he says. “My colleagues and I—especially the upperclassmen—have an important role in discussions,” he says. “We help to ease some of the unknown for the underclassmen. In the same way, I also benefit from the upperclassmen’s advice and guidance.”
Rylie Hightower, an Albuquerque native who came to UAB after earning a nursing degree in New Mexico, is one of the newer students benefiting from those discussions. At a Roadmap spring retreat, Hightower sought advice from older students. Those interactions “will help me throughout my time at UAB and beyond,” she says.
The program has “made me feel at home away from home,” Hightower says. “But it also has helped me understand that a supportive community can greatly contribute to the way I think and perform as a student and as a scientist.”
 Matthew Timberlake works with research mentor and psychiatry professor Yogesh Dwivedi.
Matthew Timberlake works with research mentor and psychiatry professor Yogesh Dwivedi.The Road Ahead
Career planning is a key component of the program. Brady, who plans to obtain a postdoctoral position following her UAB training, found Roadmap’s “postdoctoral school” to be extremely valuable. She describes it as a weeklong crash course for senior graduate students covering “everything we need to know about postdoctoral positions. Not only were we given pointers on securing the right postdoc for our interest, but we also were exposed to postdocs in fields we might not have considered.”Every student also learns about neuroscience-related job opportunities in business and industry. “Often, students are encouraged to build a career path focused on remaining in academia,” says Megan Rich, a Fairfield, Connecticut, native and UAB Graduate Biomedical Sciences student. “The program has been great about expressing other options after graduate school and that it is OK to think about them.”
By helping these students to feel empowered, capable, and supported, the Neuroscience Roadmap Scholars program will lay the groundwork for future progress in one of the fastest growing scientific fields, McMahon says. Lubin agrees, noting that diversity is crucial for continuing advancements: “Diverse groups can offer unique perspectives and unique ideas, which will push the field forward.”
• Learn more about the Neuroscience Roadmap Scholars Program, including how to apply.
• Give something and change everything for the next generation of neuroscientists by supporting the School of Medicine.
Published October 2016
Visscher Lab Makes News
By: Vicki Hixon
Published Date: Sep 15
UAB researchers use expanded computing power to accelerate big-data science by Jeff Hansen
Computing challenges are found across the UAB campus, from physics and neurology to genetics and the microbiome. Alabama’s most advanced supercomputer is now at UAB, making it possible to solve these challenges.
 Kristina Visscher is using fMRI images from several hundred brains to learn how the brain adapts after long-term changes in vision.What do the human brain, the 3 billion base-pair human genome and a tiny cube of 216 atoms have in common?
Kristina Visscher is using fMRI images from several hundred brains to learn how the brain adapts after long-term changes in vision.What do the human brain, the 3 billion base-pair human genome and a tiny cube of 216 atoms have in common?
All of them, from the tiny cube to the 3-pound human brain, create incredibly complex computing challenges for University of Alabama at Birmingham researchers, and aggressive investments in UAB’s IT infrastructure have opened new possibilities in innovation, discovery and patient care.
For example, Kristina Visscher, Ph.D., assistant professor of neurobiology, UAB School of Medicine, uses fMRI images from several hundred human brains to learn how the brain adapts after long-term changes in visual input, such as macular degeneration. Frank Skidmore, M.D., an assistant professor of neurology in the School of Medicine, studies hundreds of brain MRI images to see if they can predict Parkinson’s disease.
David Crossman, Ph.D., bioinformatics director in UAB’s Heflin Center for Genomic Science, deciphers the sequences of human genomes for patients seeking a diagnosis in UAB’s Undiagnosed Diseases Program, and he processes DNA sequencing for UAB researchers who need last-minute data for their research grant applications.
Ryoichi Kawai, Ph.D., an associate professor of physics in the UAB College of Arts and Sciences, is laying the groundwork for a better infrared laser by calculating the electronic structure for a cube made up of just 216 atoms of zinc sulfide doped with chromium or iron.
Each researcher faces a mountain range of computational challenges. Those mountains are now easier to scale with UAB’s new supercomputer — the most advanced in Alabama for speed and memory.
Using 1,000 cores, it would take two to three months of continuous computing to solve the structure, Kawai says. Both the increased number of cores and faster processing nodes in the new computer cluster will speed up that task so much that Kawai’s graduate student Kyle Bentley can investigate more different cases.
Physicist Kawai describes his computations as the most intensive on campus.“We want to capture information in an image, such as information on an individual’s brain condition,” Skidmore said. “The information we are trying capture, however, can often be difficult to see in the sea of data we collect. One brain may contain millions of bits of data in the form of ‘voxels,’ which are a bit like the pixels on your TV but in three dimensions.”
“When we map to a template,” Skidmore said, “we quadruple the data. When we ask, ‘How does this compare to a healthy brain?’ we double the data again. Then if we look across one brain or across multiple kinds of brain images, the amount of data truly explodes.”
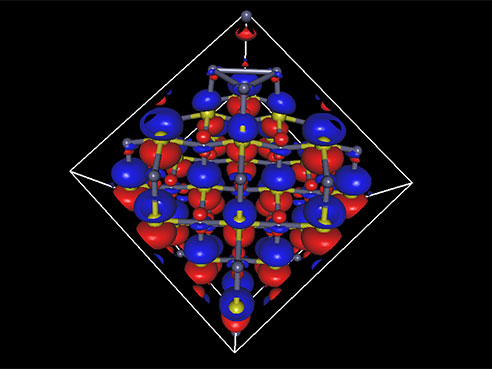 Physicist Ryoichi Kawai's complex calculations of the electronic structure of a cube of atoms are laying the groundwork for better lasers.“This is made even more complex by the fact that a given image can include more than three dimensions of information,” he said. “One type of image we use generates 5-dimensional brain maps. Since we can’t see in five dimensions, we ask the computer to work in these higher-dimensional spaces to help us pull the information out of the data.”
Physicist Ryoichi Kawai's complex calculations of the electronic structure of a cube of atoms are laying the groundwork for better lasers.“This is made even more complex by the fact that a given image can include more than three dimensions of information,” he said. “One type of image we use generates 5-dimensional brain maps. Since we can’t see in five dimensions, we ask the computer to work in these higher-dimensional spaces to help us pull the information out of the data.”
To look at the adult brain’s plasticity — the ability to change function and structure through new synaptic connections — Visscher studies visual processing.
“Because we look at spatial and temporal data, the number of pieces of information is huge — gigabytes per subject,” she said. “We need to do correlations on all the data points at the same time. To get faster, we optimize the data analysis with a lot of feedback. Then we run what we learned from one brain on a hundred brains.”
The new supercomputer “will drastically improve our computing capacity from what we have had,” Crossman said. “With the old cluster, my job might sit in a queue for a couple of weeks. With undiagnosed diseases, that is not acceptable because that’s a patient.”“And I cannot tell a researcher, ‘I’m sorry, we won’t meet that grant deadline,’” Crossman said. “You know, science can’t stop.”
The data floodgates of genomics burst open about a dozen years ago with the arrival of next-generation, high-throughput sequencing, says Elliot Lefkowitz, Ph.D., director of Informatics for the UAB Center for Clinical and Translational Science. Lefkowitz has been serving the bioinformatics needs of the UAB Center for AIDS Research for 25 years, and now also handles bioinformatics for the UAB Microbiome Facility. His team has grown to five bioinformaticians and several programmers.
“We deal with billions of sequences when we do a run through the DNA sequencing machine,” Lefkowitz said. “We need to compare every one of the billion ‘reads’ (the 50- to 300-base sequence of a short piece of DNA) to every other one. With high-performance computing and thousands of nodes, each one does part of the job.”
“In not too many years,” Lefkowitz speculated, “we will be sequencing every patient coming into University Hospital.”
Changes like that mean ever-increasing computer demands.
“Biomedical research,” Crossman said, “now is big data.”
Computing challenges are found across the UAB campus, from physics and neurology to genetics and the microbiome. Alabama’s most advanced supercomputer is now at UAB, making it possible to solve these challenges.
 Kristina Visscher is using fMRI images from several hundred brains to learn how the brain adapts after long-term changes in vision.What do the human brain, the 3 billion base-pair human genome and a tiny cube of 216 atoms have in common?
Kristina Visscher is using fMRI images from several hundred brains to learn how the brain adapts after long-term changes in vision.What do the human brain, the 3 billion base-pair human genome and a tiny cube of 216 atoms have in common?All of them, from the tiny cube to the 3-pound human brain, create incredibly complex computing challenges for University of Alabama at Birmingham researchers, and aggressive investments in UAB’s IT infrastructure have opened new possibilities in innovation, discovery and patient care.
For example, Kristina Visscher, Ph.D., assistant professor of neurobiology, UAB School of Medicine, uses fMRI images from several hundred human brains to learn how the brain adapts after long-term changes in visual input, such as macular degeneration. Frank Skidmore, M.D., an assistant professor of neurology in the School of Medicine, studies hundreds of brain MRI images to see if they can predict Parkinson’s disease.
David Crossman, Ph.D., bioinformatics director in UAB’s Heflin Center for Genomic Science, deciphers the sequences of human genomes for patients seeking a diagnosis in UAB’s Undiagnosed Diseases Program, and he processes DNA sequencing for UAB researchers who need last-minute data for their research grant applications.
Ryoichi Kawai, Ph.D., an associate professor of physics in the UAB College of Arts and Sciences, is laying the groundwork for a better infrared laser by calculating the electronic structure for a cube made up of just 216 atoms of zinc sulfide doped with chromium or iron.
Each researcher faces a mountain range of computational challenges. Those mountains are now easier to scale with UAB’s new supercomputer — the most advanced in Alabama for speed and memory.
The tiny cube
The 216-atom electronic structure problem “is the largest calculation on campus, by far,” Kawai said. “If you give me 2,000 cores, I can use them. If you give me 10,000 cores, I can use them all without losing efficiency.” (UAB's new supercomputer has 2,304 cores.)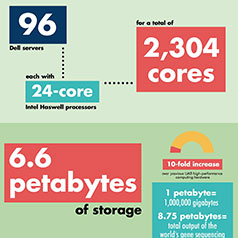 Learn more about UAB’s new supercomputer in this story Learn more about UAB’s new supercomputer in this story |
Brains
In their research, both Skidmore and Visscher have to compare brains with other brains. Because each brain differs somewhat in size, shape and surface folds, every brain has to be mapped onto a template to allow comparisons.Physicist Kawai describes his computations as the most intensive on campus.“We want to capture information in an image, such as information on an individual’s brain condition,” Skidmore said. “The information we are trying capture, however, can often be difficult to see in the sea of data we collect. One brain may contain millions of bits of data in the form of ‘voxels,’ which are a bit like the pixels on your TV but in three dimensions.”
“When we map to a template,” Skidmore said, “we quadruple the data. When we ask, ‘How does this compare to a healthy brain?’ we double the data again. Then if we look across one brain or across multiple kinds of brain images, the amount of data truly explodes.”
 Physicist Ryoichi Kawai's complex calculations of the electronic structure of a cube of atoms are laying the groundwork for better lasers.“This is made even more complex by the fact that a given image can include more than three dimensions of information,” he said. “One type of image we use generates 5-dimensional brain maps. Since we can’t see in five dimensions, we ask the computer to work in these higher-dimensional spaces to help us pull the information out of the data.”
Physicist Ryoichi Kawai's complex calculations of the electronic structure of a cube of atoms are laying the groundwork for better lasers.“This is made even more complex by the fact that a given image can include more than three dimensions of information,” he said. “One type of image we use generates 5-dimensional brain maps. Since we can’t see in five dimensions, we ask the computer to work in these higher-dimensional spaces to help us pull the information out of the data.”To look at the adult brain’s plasticity — the ability to change function and structure through new synaptic connections — Visscher studies visual processing.
“Because we look at spatial and temporal data, the number of pieces of information is huge — gigabytes per subject,” she said. “We need to do correlations on all the data points at the same time. To get faster, we optimize the data analysis with a lot of feedback. Then we run what we learned from one brain on a hundred brains.”
UAB customers
Crossman deciphers the sequences of human genomes for patients seeking a diagnosis, and he processes DNA sequencing for UAB researchers. Providing excellent customer service to his clients is vital, Crossman says, and it takes computer power to crunch genome sequencing data for those researchers, physicians and patients.
Managing traffic on the new UAB computer clusterSize:
|
The data floodgates of genomics burst open about a dozen years ago with the arrival of next-generation, high-throughput sequencing, says Elliot Lefkowitz, Ph.D., director of Informatics for the UAB Center for Clinical and Translational Science. Lefkowitz has been serving the bioinformatics needs of the UAB Center for AIDS Research for 25 years, and now also handles bioinformatics for the UAB Microbiome Facility. His team has grown to five bioinformaticians and several programmers.
“We deal with billions of sequences when we do a run through the DNA sequencing machine,” Lefkowitz said. “We need to compare every one of the billion ‘reads’ (the 50- to 300-base sequence of a short piece of DNA) to every other one. With high-performance computing and thousands of nodes, each one does part of the job.”
“In not too many years,” Lefkowitz speculated, “we will be sequencing every patient coming into University Hospital.”
Changes like that mean ever-increasing computer demands.
“Biomedical research,” Crossman said, “now is big data.”
McKnight Poster Session
By: Vicki Hixon
Published Date: Aug 12
Hosted by:
Evelyn F. McKnight Brain Institute, The University of Alabama at Birmingham
Evelyn F. McKnight Brain Institute, The University of Arizona
Evelyn F. & William L. McKnight Brain Institute, The University of Florida
Evelyn F. McKnight Brain Institute, The University of Miami
Sponsored by:
McKnight Brain Research Foundation
Established by Evelyn F. McKnight
You are cordially invited to the
Ninth Annual
McKnight Brain Research Foundation
Poster Reception
Sunday, November 13, 2016
6:30 - 8:30 p.m.
Hilton San Diego Bayfront
One Park Boulevard
San Diego, CA 92101
Hosted by:
Evelyn F. McKnight Brain Institute, The University of Alabama at Birmingham
Evelyn F. McKnight Brain Institute, The University of Arizona
Evelyn F. & William L. McKnight Brain Institute, The University of Florida
Evelyn F. McKnight Brain Institute, The University of Miami
Sponsored by:
McKnight Brain Research Foundation
Established by Evelyn F. McKnight
Art Wanted
By: Vicki Hixon
Published Date: Aug 04


Discovery may lead to a treatment to slow Parkinson's disease
By: Vicki Hixon
Published Date: Jul 27
Discovery may lead to a treatment to slow Parkinson’s disease by Jeff Hansen
Researchers have found that an interaction between a mutant gene and alpha synuclein in neurons leads to hallmark pathologies seen in Parkinson’s disease, findings that may lead to new mechanisms and targets for neuroprotection.
 Laura A. Volpicelli-DaleyUsing a robust model for Parkinson’s disease, University of Alabama at Birmingham researchers and colleagues have discovered an interaction in neurons that contributes to Parkinson’s disease, and they have shown that drugs now under development may block the process.
Laura A. Volpicelli-DaleyUsing a robust model for Parkinson’s disease, University of Alabama at Birmingham researchers and colleagues have discovered an interaction in neurons that contributes to Parkinson’s disease, and they have shown that drugs now under development may block the process.
The research team has shown that the most common genetic cause of Parkinson’s disease — a mutant LRRK2 kinase enzyme — contributes to the formation of inclusions in neurons, resembling one of the hallmark pathologies seen in Parkinson’s disease. These inclusions are made up of aggregated alpha synuclein protein, which — the research also shows — can be prevented from forming by using two LRRK2 kinase inhibitor drugs now being developed for clinical use.
The interaction between mutant LRRK2 kinase and alpha-synuclein “may uncover new mechanisms and targets for neuroprotection,” the researchers write in a recent Journal of Neuroscience paper. “These results demonstrate that alpha-synuclein inclusion formation in neurons can be blocked and that novel therapeutic compounds targeting this process by inhibiting LRRK2 kinase activity may slow progression of Parkinson’s disease-associated pathology.”
The potential clinical applications for novel neuroprotection strategies in LRRK2-linked Parkinson’s need to be tested in other preclinical models of Parkinson’s disease, say the researchers, led by corresponding author Laura A. Volpicelli-Daley, Ph.D., and senior author Andrew B. West, Ph.D., Center for Neurodegeneration and Experimental Therapeutics, UAB Department of Neurology.
“These data give us hope for the clinical potential of LRRK2 kinase inhibitors as effective therapies for Parkinson’s disease,” Volpicelli-Daley said. “The LRRK2 kinase inhibitors may inhibit the spread of pathologic alpha-synuclein, not only in patients with LRRK2 mutations, but in all Parkinson’s disease patients. Future studies to validate the safety and efficacy of the LRRK2 inhibitors will be necessary before testing the inhibitors in human clinical trials.”
Besides Parkinson’s disease, alpha-synuclein also plays a central role in development of dementia with Lewy bodies and multiple system atrophy, and it is associated with Alzheimer’s disease and other neurodegenerative disorders.
The Parkinson’s disease model developed by Volpicelli-Daley applies very low concentrations of pre-formed fibrils of alpha-synuclein to in vitro or in vivo neurons. This causes formation of modified alpha-synuclein inclusions that share morphology with those found in the Parkinson’s disease brain after death.
They used this model to test the effects of neuron expression of the mutant LRRK2 (“lark two”) kinase, G2019S-LRRK2, on the formation of the inclusion pathology.
They found that:
 Andrew B. WestIn fluorescence-recovery-after-photobleaching experiments, they found there was a larger pool of mobile alpha-synuclein, as opposed to membrane-bound alpha-synuclein, in neurons that expressed G2019S-LRRK2. Recent work by others has shown that mobile alpha-synuclein is prone to misfolding and aggregation, so the researchers hypothesize that the G2019S-LRRK2 mutation may contribute to Parkinson’s susceptibility by boosting the amounts of mobile alpha-synuclein in neurons.
Andrew B. WestIn fluorescence-recovery-after-photobleaching experiments, they found there was a larger pool of mobile alpha-synuclein, as opposed to membrane-bound alpha-synuclein, in neurons that expressed G2019S-LRRK2. Recent work by others has shown that mobile alpha-synuclein is prone to misfolding and aggregation, so the researchers hypothesize that the G2019S-LRRK2 mutation may contribute to Parkinson’s susceptibility by boosting the amounts of mobile alpha-synuclein in neurons.
Besides Volpicelli-Daley and West, co-authors of the paper “G2019S-LRRK2 expression augments alpha-synuclein sequestration into inclusions in neurons” are Hisham Abdelmotilib, Zhiyong Liu, Lindsay Stoyka, João Paulo Lima Daher and Kyle Fraser, all of the Center for Neurodegeneration and Experimental Therapeutics, UAB Department of Neurology; Austen J. Milnerwood, Centre for Applied Neurogenetics, University of British Columbia; Vivek K. Unni, Jungers Center for Neurosciences Research and Parkinson Center of Oregon, Oregon Health & Science University; Warren D. Hirst, Pfizer Neuroscience and Pain Research Unit, Cambridge, Massachusetts; Zhenyu Yue, Departments of Neurology and Neuroscience, Icahn School of Medicine at Mount Sinai; Hien T. Zhao, Ionis Pharmaceuticals, Carlsbad, California; and Richard E. Kennedy, Comprehensive Center for Healthy Aging and Division of Gerontology, Geriatrics, and Palliative Care, UAB Department of Medicine.
Grants to fund this work came from the American Parkinson’s Disease Association, the Michael J. Fox Foundation LEAPS Award and the National Institutes of Health NS064934.
Volpicelli-Daley is an assistant professor in the Department of Neurology.
West is co-director of the Center for Neurodegeneration and Experimental Therapeutics, and the John A. and Ruth R. Jurenko Professor of Neurology at UAB.
 Laura A. Volpicelli-DaleyUsing a robust model for Parkinson’s disease, University of Alabama at Birmingham researchers and colleagues have discovered an interaction in neurons that contributes to Parkinson’s disease, and they have shown that drugs now under development may block the process.
Laura A. Volpicelli-DaleyUsing a robust model for Parkinson’s disease, University of Alabama at Birmingham researchers and colleagues have discovered an interaction in neurons that contributes to Parkinson’s disease, and they have shown that drugs now under development may block the process.The research team has shown that the most common genetic cause of Parkinson’s disease — a mutant LRRK2 kinase enzyme — contributes to the formation of inclusions in neurons, resembling one of the hallmark pathologies seen in Parkinson’s disease. These inclusions are made up of aggregated alpha synuclein protein, which — the research also shows — can be prevented from forming by using two LRRK2 kinase inhibitor drugs now being developed for clinical use.
The interaction between mutant LRRK2 kinase and alpha-synuclein “may uncover new mechanisms and targets for neuroprotection,” the researchers write in a recent Journal of Neuroscience paper. “These results demonstrate that alpha-synuclein inclusion formation in neurons can be blocked and that novel therapeutic compounds targeting this process by inhibiting LRRK2 kinase activity may slow progression of Parkinson’s disease-associated pathology.”
The potential clinical applications for novel neuroprotection strategies in LRRK2-linked Parkinson’s need to be tested in other preclinical models of Parkinson’s disease, say the researchers, led by corresponding author Laura A. Volpicelli-Daley, Ph.D., and senior author Andrew B. West, Ph.D., Center for Neurodegeneration and Experimental Therapeutics, UAB Department of Neurology.
“These data give us hope for the clinical potential of LRRK2 kinase inhibitors as effective therapies for Parkinson’s disease,” Volpicelli-Daley said. “The LRRK2 kinase inhibitors may inhibit the spread of pathologic alpha-synuclein, not only in patients with LRRK2 mutations, but in all Parkinson’s disease patients. Future studies to validate the safety and efficacy of the LRRK2 inhibitors will be necessary before testing the inhibitors in human clinical trials.”
Besides Parkinson’s disease, alpha-synuclein also plays a central role in development of dementia with Lewy bodies and multiple system atrophy, and it is associated with Alzheimer’s disease and other neurodegenerative disorders.
 Primary hippocampal neurons from mice expressing G2019S-LRRK2. The neurons were treated with alpha-synuclein fibrils, and 18 days later immunofluorescence was performed. The magenta shows phospho-alpha-synuclein inclusions in the cell bodies and throughout the axons, which are visualized as green.Research details
Primary hippocampal neurons from mice expressing G2019S-LRRK2. The neurons were treated with alpha-synuclein fibrils, and 18 days later immunofluorescence was performed. The magenta shows phospho-alpha-synuclein inclusions in the cell bodies and throughout the axons, which are visualized as green.Research details
The Parkinson’s disease model developed by Volpicelli-Daley applies very low concentrations of pre-formed fibrils of alpha-synuclein to in vitro or in vivo neurons. This causes formation of modified alpha-synuclein inclusions that share morphology with those found in the Parkinson’s disease brain after death.They used this model to test the effects of neuron expression of the mutant LRRK2 (“lark two”) kinase, G2019S-LRRK2, on the formation of the inclusion pathology.
They found that:
- G2019S-LRRK2 enhanced alpha-synuclein inclusions in primary hippocampal neurons from the hippocampus region of the brain, 18 days after fibril exposure, as compared with neurons that over-expressed normal LRRK2.
- The effects of G2019S-LRRK2 expression in the fibril-exposed neurons were lessened by very low concentrations of potent and selective preclinical drugs that inhibit LRRK2 kinase. This suggested that the kinase activity of G2019S-LRRK2, which adds a phosphate onto target proteins, underlies the faster formation of pathologic alpha-synuclein inclusions.
- G2019S-LRRK2 expression enhanced alpha-synuclein inclusion formation in dopamine neurons from the region of the brain called the substantia nigra pars compacta. The substantia nigra pars compacta is the area of the brain that dies in Parkinson’s disease, so this experiment further supports a link between the G2019S-LRRK2 mutation and Parkinson’s pathogenesis.
 Andrew B. WestIn fluorescence-recovery-after-photobleaching experiments, they found there was a larger pool of mobile alpha-synuclein, as opposed to membrane-bound alpha-synuclein, in neurons that expressed G2019S-LRRK2. Recent work by others has shown that mobile alpha-synuclein is prone to misfolding and aggregation, so the researchers hypothesize that the G2019S-LRRK2 mutation may contribute to Parkinson’s susceptibility by boosting the amounts of mobile alpha-synuclein in neurons.
Andrew B. WestIn fluorescence-recovery-after-photobleaching experiments, they found there was a larger pool of mobile alpha-synuclein, as opposed to membrane-bound alpha-synuclein, in neurons that expressed G2019S-LRRK2. Recent work by others has shown that mobile alpha-synuclein is prone to misfolding and aggregation, so the researchers hypothesize that the G2019S-LRRK2 mutation may contribute to Parkinson’s susceptibility by boosting the amounts of mobile alpha-synuclein in neurons.Besides Volpicelli-Daley and West, co-authors of the paper “G2019S-LRRK2 expression augments alpha-synuclein sequestration into inclusions in neurons” are Hisham Abdelmotilib, Zhiyong Liu, Lindsay Stoyka, João Paulo Lima Daher and Kyle Fraser, all of the Center for Neurodegeneration and Experimental Therapeutics, UAB Department of Neurology; Austen J. Milnerwood, Centre for Applied Neurogenetics, University of British Columbia; Vivek K. Unni, Jungers Center for Neurosciences Research and Parkinson Center of Oregon, Oregon Health & Science University; Warren D. Hirst, Pfizer Neuroscience and Pain Research Unit, Cambridge, Massachusetts; Zhenyu Yue, Departments of Neurology and Neuroscience, Icahn School of Medicine at Mount Sinai; Hien T. Zhao, Ionis Pharmaceuticals, Carlsbad, California; and Richard E. Kennedy, Comprehensive Center for Healthy Aging and Division of Gerontology, Geriatrics, and Palliative Care, UAB Department of Medicine.
Grants to fund this work came from the American Parkinson’s Disease Association, the Michael J. Fox Foundation LEAPS Award and the National Institutes of Health NS064934.
Volpicelli-Daley is an assistant professor in the Department of Neurology.
West is co-director of the Center for Neurodegeneration and Experimental Therapeutics, and the John A. and Ruth R. Jurenko Professor of Neurology at UAB.
Research Grant Proposals
By: Vicki Hixon
Published Date: Jul 25
The Civitan International Research Center Research Grants for Emerging Scholars Request for Proposals
Civitan Travel Awards
Day Published in Nature Communications
By: Vicki Hixon
Published Date: Jul 07
Extra-coding RNAs regulate DNA methylation in the adult brain
Pozzo-Miller Lab and Dudek Lab Collaboration
By: Kerry Gorelick
Published Date: Jun 23
Perineuronal Nets Suppress Plasticity of Excitatory Synapses on CA2 Pyramidal Neurons
Kelly E. Carstens, Mary L. Phillips, Lucas Pozzo-Miller, Richard J. Weinberg, and Serena M. Dudek
The Journal of Neuroscience, 8 June 2016, 36(23):6312-6320; doi:10.1523/JNEUROSCI.0245-16.2016 FEATURED ARTICLE COVER ILLUSTRATION
http://www.jneurosci.org/content/36/23/6312.abstract
Kelly E. Carstens, Mary L. Phillips, Lucas Pozzo-Miller, Richard J. Weinberg, and Serena M. Dudek
The Journal of Neuroscience, 8 June 2016, 36(23):6312-6320; doi:10.1523/JNEUROSCI.0245-16.2016 FEATURED ARTICLE COVER ILLUSTRATION
http://www.jneurosci.org/content/36/23/6312.abstract
 Perineuronal Nets Suppress Plasticity of Excitatory Synapses on CA2 Pyramidal Neurons
Perineuronal Nets Suppress Plasticity of Excitatory Synapses on CA2 Pyramidal Neurons